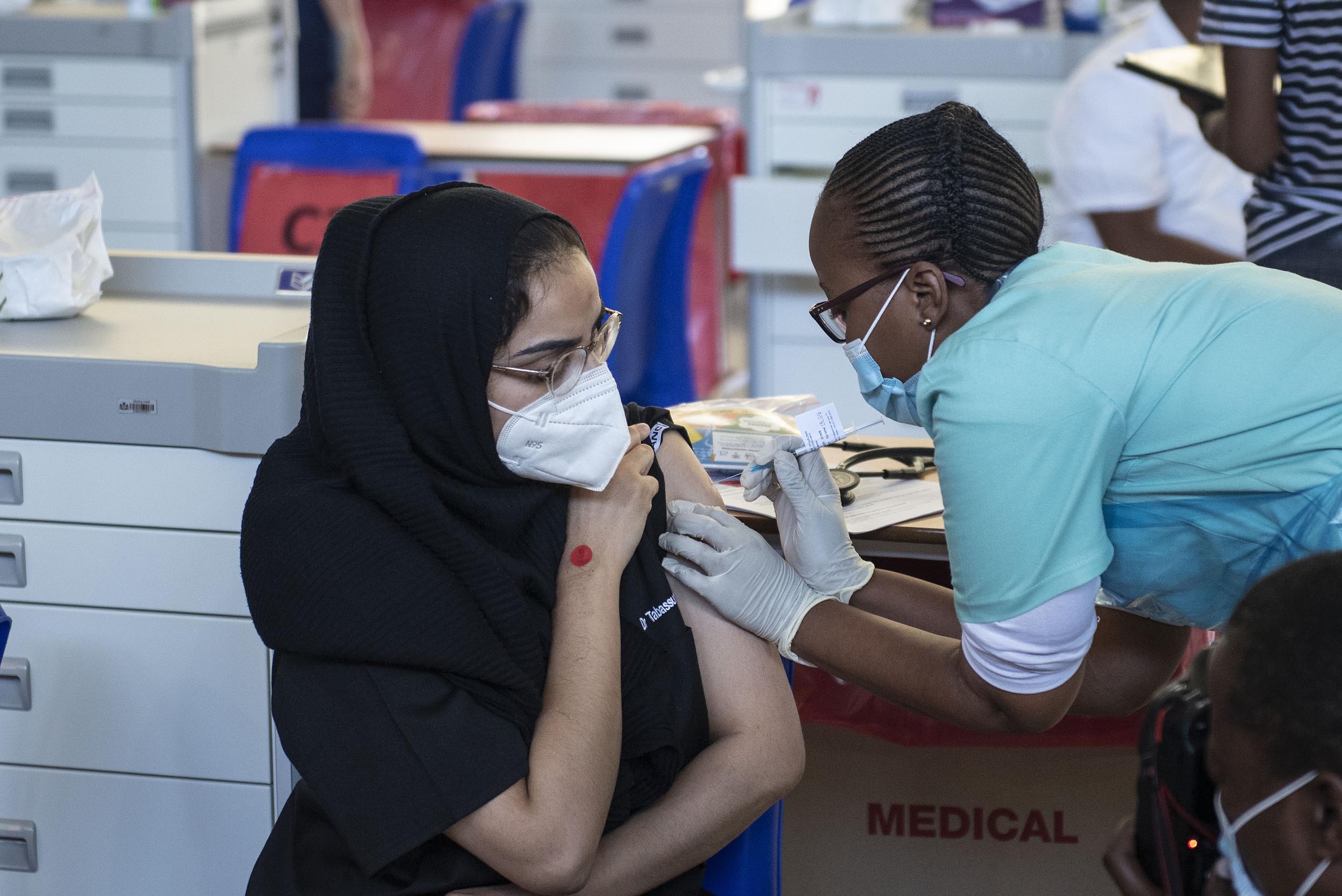
South Africans’ chances against the imminent third wave of Covid-19 infections may be worse off because of the halting of the Johnson & Johnson vaccine Janssen, says one of the doctors involved in trialling the drug.
Dr Hugo Tempelman, CEO of the Ndlovu Care Group which conducted trials for the vaccine last year, says though it’s understandable why first world countries can afford to halt the rollout of the vaccine, the same couldn’t be said about South Africa and other developing nations.
Weighing the risks of adverse effects from the vaccine against those faced by South Africa, should it fail to vaccinate enough people on time, Tempelman’s call is that the decision was premature.
Also Read: SAHPRA recommends lift of J&J rollout suspension
“If all the countries are stopping then you cannot say as a country, I continue. But all those countries who stop have alternatives like Moderna or Pfizer and other vaccines and we don’t,” says Tempelman.
“We have taken a first world decision in a third world environment and a low-income environment and I don’t think this decision should be the same in both scenarios.”
The National Education, Health and Allied Workers’ Union (Nehawu) continued its support of the halting of the Sisonke Program through which the healthcare workers were being vaccinated.
This despite being concerned that the winter months and a possible third wave are approaching with no sign of the vaccine rollout picking up speed.
Nehawu supported the move last week, calling on government to ensure that those who have been administered the drug are monitored for side-effects.
Senior Researcher for the Council for Scientific and Industrial Research (CSIR) Ridhawan Sulaiman suggested in a tweet on Sunday that South Africa was headed towards an ‘Easter wave’ driven by a resurgence in three of the smaller provinces, namely the free state, Northern Cape and the North West Province.
Motivating this argument, he noted there had been a 35% increase in reported cases of Covid-19 this week and only a 19% increase in testing.
Time for our Sunday update of #COVID19 in SA????????
Clear signs of an “Easter wave” (on cue), driven by a resurgence in 3 of the smaller provinces: NC, FS and NW ⚠️????
• Cases +35% ⬆️
• Tests +19% ⬆️
• Test positivity rate +14% ⬆️
• Hospitalisations +2% ⬆️
• Deaths +51% ⬆️ pic.twitter.com/Aoxuur0KER— Ridhwaan Suliman (@rid1tweets) April 18, 2021
But this weekend the South African Health Products Regulatory Authority (Sahpra) recommended the program be resumed.
The agency had engagements with the the Sisonke Phase 3B Implementation Study team and Janssen Pharmaceutica to discuss the safety data reported from the Sisonke study, following administration of the Covid-19 vaccine Janssen.
These events take place as South Africa continues to support a call for pharmaceutical companies and the World Trade Organisation (WTO) to waver intellectual property rights for Covid-19 vaccines and treatment.
Last week health minister Zweli Mkhize told parliament that J&J refused to sign off on their order of the doses until certain terms were agreed to, including greater support from the state.
Tempelman has iterated that South Africa was better off brokering a deal for a zero-profit pricing structure in order to make the vaccine affordable. The company plans to manufacture over 300 million doses of the vaccine on South African shores.
“If [the rollout] continued and you were to extrapolate the figures, 11 cases of blood clots from seven million -that should mean that for South Africa that we would have to swallow 90 deaths. We could expect about 100 deaths should we continue,” says Tempelman.
“Daily at the moment we have about 100 deaths from Covid-19. I think I should not defend the decision because we are heading for a third wave and we could have vaccinations for front-line healthcare workers and I think that should have been a major factor in the decision making.”
Nehawu spokesperson Khaya Xaba is cautious in his noting of Sahpra’s recommendation. Fears of serous side effects still abound.
“We note their recommendation, however more stringent tests will have to be done in order to manage any adverse side effects. Of course there must be measures put in place to accompany the lifting of the pause if government decides to do so to monitor the possibility of side effects.”
-Simnikiweh@citizen.co.za