As more people become eligible to receive a coronavirus vaccine many are asking what side-effects they should expect, and if there are differences between the side-effects of the vaccines. The short answer to both questions is yes – the details are below – though any discomfort pales in comparison with contracting Covid-19.
We used clinical trial data gathered by the US Food and Drug Administration (FDA) to explore the kinds of side-effects most commonly associated with the three vaccines currently authorized for emergency use in the US. Those vaccines were developed by Pfizer and BioNTech, Moderna and the National Institute of Allergy and Infectious Disease (Niaid) and most recently by Johnson & Johnson.
What are the common side-effects?
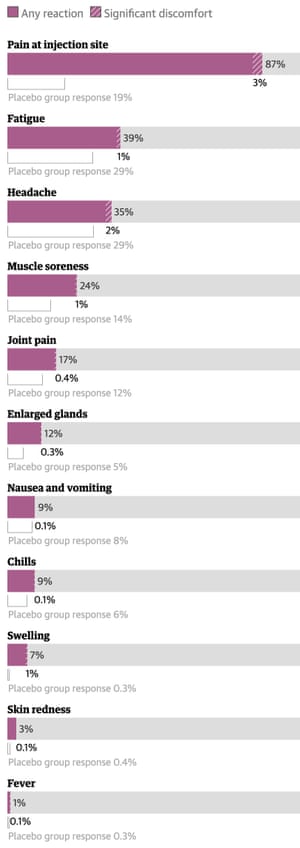
For all the vaccines, the most common side-effects include:
Less common side-effects can also include nausea, chills and fever. The vast majority of symptoms cause discomfort, but not a total disruption of your daily habits.
Are Covid-19 vaccines safe?
Yes. Their safety is tested in large trials of tens of thousands of people, then the FDA and the Centers for Disease Control and Prevention (CDC) continue to monitor vaccine safety data, including side-effects, after the vaccines are authorized. These are sometimes referred to as phase IV trials. That monitoring goes hand-in-hand with reporting through several vaccine safety registries.
These ongoing studies can help identify the rarest of side-effects, and pinpoint people who may have special sensitivities to the vaccine, such as a potential for an allergic reaction.
One of the key numbers included in the graphs below is the rate of people who experienced side-effects after receiving a “placebo”, or an injection of saline instead of the vaccine. People involved in the trials did not know whether or not they received the vaccine. This helps researchers understand the background rate of these side-effects in the population.
Moderna vaccine side-effects
Formally called mRNA-1273, this vaccine was developed by Moderna in partnership with the Niaid, but most people simply know it as the Moderna vaccine, which is a two-dose regimen spaced 28 days apart.
A clinical trial involving more than 30,000 participants across 99 sites in the US found the vaccine was safe and effective, and protected people against Covid 94.1% of the time. Among those trial participants, 15,168 people received the vaccine and the rest received a placebo.
Moderna dose 1 side-effects
We used results from the vaccine’s trials to describe how likely it is for people aged 18 to 64 to experience a given side-effect within one week of a dose of the vaccine. On average, these symptoms cleared up within three days, and often less.