An Emergency Use Authorization (EUA) request has been submitted to the US Food and Drug Administration (FDA) for the adjuvanted Novavax COVID-19 vaccine as a homologous and heterologous booster to prevent COVID-19 caused by SARS-CoV-2 in adults 18 years of age and older.
The Novavax COVID-19 vaccine is engineered from the genetic sequence of SARS-CoV-2 using the company’s recombinant nanoparticle technology to generate antigen derived from the coronavirus spike protein. The vaccine contains the company’s saponin-based Matrix-M™ adjuvant technology to enhance and prolong immune responses.
The submission is supported by data from the randomized, placebo-controlled, phase 3 PREVENT-19 trial (ClinicalTrials.gov Identifier: NCT04611802), which evaluated the efficacy, safety, and immunogenicity of the Novavax COVID-19 vaccine in participants 18 years of age and older in the United States and Mexico. Participants received a booster dose of the Novavax COVID-19 vaccine at least 6 months after the primary 2-dose series. Findings showed that the booster dose elicited robust antibody responses comparable to or exceeding levels associated with the efficacy data in the primary series of the vaccine.
Continue Reading
Additionally, the EUA submission included data from the multicenter, randomized, controlled, phase 2 COV-BOOST trial, which showed that a heterologous booster dose of the Novavax COVID-19 vaccine produced a significant antibody response.
As for safety, the Novavax COVID-19 vaccine was associated with local and systemic reactions with a median duration of approximately 2 days. The incidence of medically attended adverse events, potentially immune-mediated medical conditions, and severe adverse events occurred infrequently following the booster dose.
“It’s important for people to have a choice as they evaluate how to stay protected against COVID-19, and boosters are an invaluable tool to build upon immunity obtained from previous vaccinations,” said Stanley C. Erck, President and CEO of Novavax. “Based on the data presented to the FDA’s VRBPAC and the CDC ACIP, we believe our vaccine offers a broad, long-lasting immune response against a range of variants.”
The FDA recently granted EUA to the adjuvanted Novavax COVID-19 vaccine as a primary 2-dose series for the prevention of COVID-19 caused by SARS-CoV-2 in individuals 18 years of age and older. The vaccine is administered intramuscularly as a 2-dose primary series (each 0.5 mL), separated by 3 weeks.
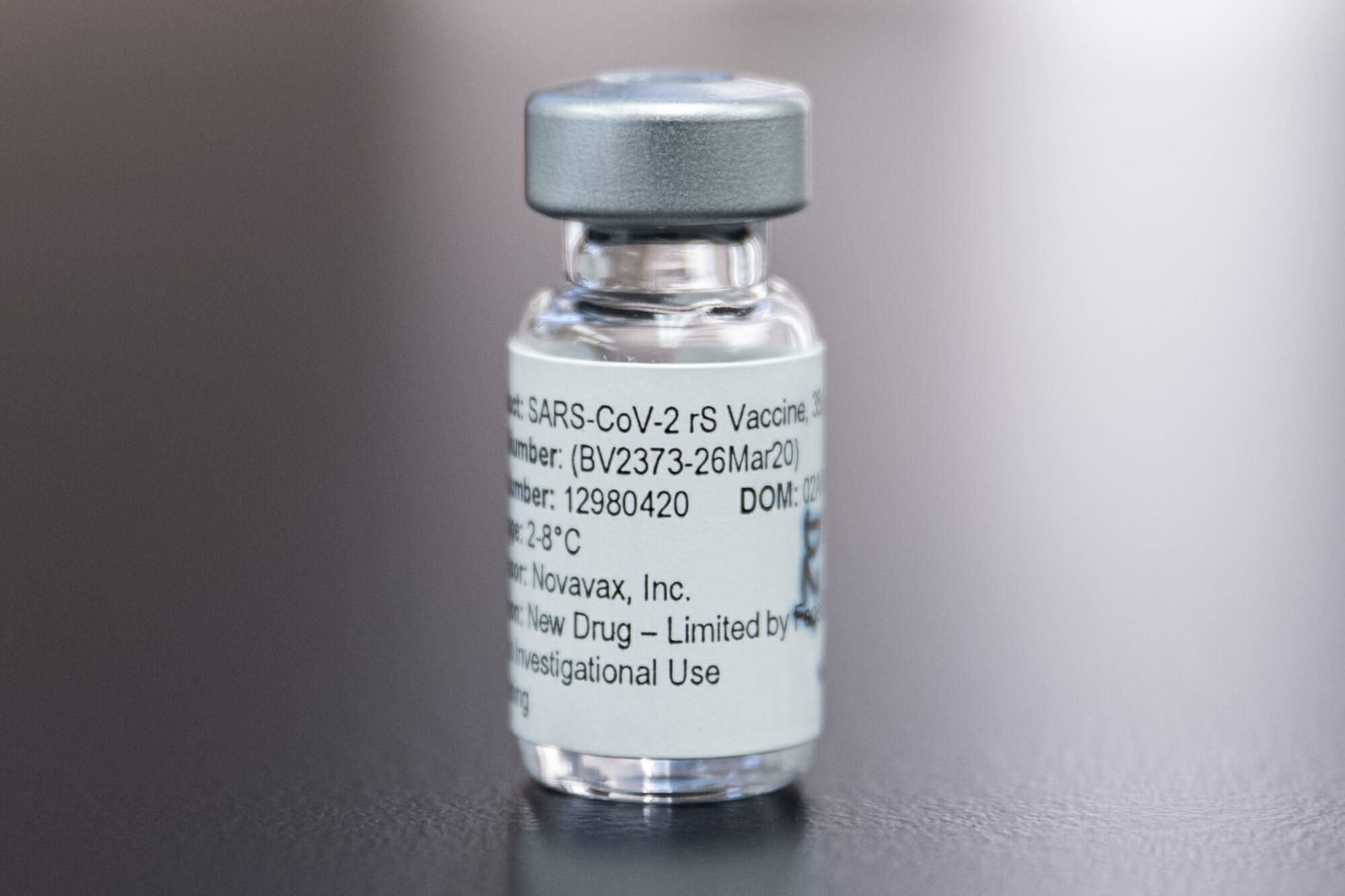
The Novavax COVID-19 vaccine is supplied as a suspension in a carton containing 10 multidose vials; each vial contains 10 doses of 0.5 mL each.
Reference
Novavax submits application to the US FDA for Emergency Use Authorization for Novavax COVID-19 Vaccine, Adjuvanted as a booster in adults aged 18 and older. News release. Novavax, Inc. Accessed August 15, 2022. https://www.prnewswire.com/news-releases/novavax-submits-application-to-the-us-fda-for-emergency-use-authorization-for-novavax-covid-19-vaccine-adjuvanted-as-a-booster-in-adults-aged-18-and-older-301605447.html
This article originally appeared on MPR
this content first appear on medical bag

One more thing. I believe that there are numerous travel insurance web sites of reliable companies that allow you enter your trip details and find you the prices. You can also purchase an international holiday insurance policy on the net by using your current credit card. All you have to do will be to enter your travel specifics and you can see the plans side-by-side. You only need to find the program that suits your capacity to pay and needs after which use your credit card to buy it. Travel insurance on the internet is a good way to take a look for a respected company for international holiday insurance. Thanks for revealing your ideas. Amado Eisinger
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.