Australia’s Covid-19 rollout is in disarray after the announcement that the locally-manufactured AstraZeneca vaccine, the backbone of Australia’s rollout program, should be avoided for those aged under 50. The announcement is causing a significant “recalibration” of the nation’s plans to protect itself from Covid-19.
It bears repeating: the AstraZeneca vaccine is highly effective and is safe for the overwhelming majority of patients. Fatal blood clotting is extremely rare.
But what are our other vaccine options, given this latest advice? And how far off is Australia from accessing alternative vaccine candidates?
The Pfizer vaccine
Type: mRNA
Supply: 40m doses on order, 870,000 delivered
Approval status: approved for use by the TGA
Source: manufactured in the US and Europe
An mRNA vaccine, the Pfizer vaccine has proven safe and highly effective. It has finished its clinical trials, has been approved by the Therapeutic Goods Administration, and is in widespread use across the globe.
Australia, though, has no capability to manufacture the Pfizer vaccine onshore, so we’ve been reliant on imports in a world of increasing vaccine protectionism.
The government has ordered 40m Pfizer doses, enough for 20m people.
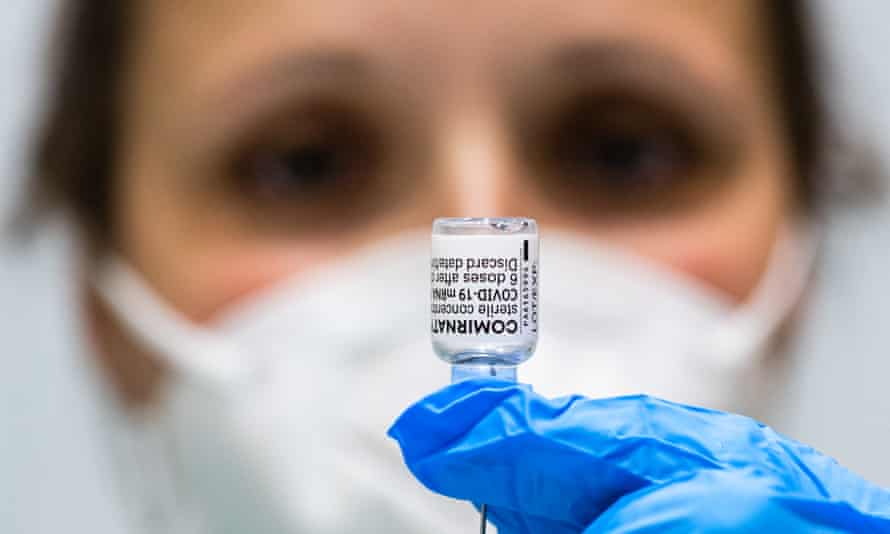
So far, the government says the imports have been consistent. About 110,000 to 150,000 Pfizer doses are arriving in each shipment, the government says, and we have received in total more than 870,000. Those doses have mainly been used in aged care and for frontline health workers.
Health minister Greg Hunt says he has received assurances from Pfizer’s Australian chief that the first 20m doses will arrive this year.
“I spoke directly with the Australian CEO of Pfizer, Anne Harris, in the last 24 hours, and she has reconfirmed Pfizer’s advice that those full 20 million doses would be available during the course of 2021 on their current plan and current schedule,” he said on Thursday.
The government previously indicated it is also in discussions with Pfizer to secure more supplies and speed up deliveries. On Friday, it announced it has secured a further 20m doses, but they won’t be available until late this year.
Novavax
Type: protein vaccine
Supply: 50m on order, none yet delivered
Approval status: phase three clinical trials
Source: Europe
Australia has ordered 50m doses of Novavax’s Covid-19 vaccine. Recent studies suggest it is 100% effective against severe disease and 96.4% effective against mild and moderate illness. That effectiveness drops off somewhat with new Covid-19 variants.
So it looks promising.
But Australia is still months away from being able to use Novavax.
The vaccine is still undergoing phase three clinical trials and is yet to receive TGA approval for use here. On top of that, the company is expected to prioritise securing approvals from US and UK regulators.
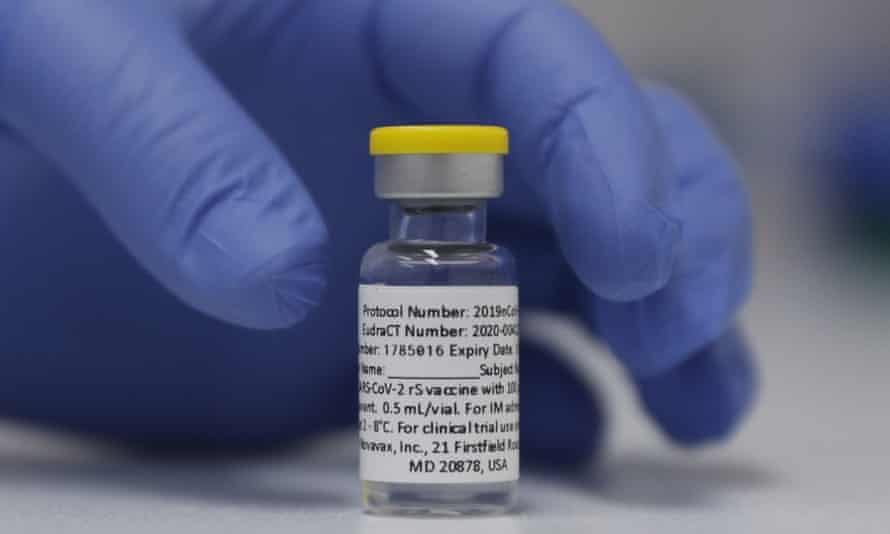
Hunt said on Thursday that he had spoken with the company’s international executive vice-president this week and they indicated that the first doses for Australia are “due in quarter three of this year”.
There appears to be no set timeline for delivery baked into the Novavax contract.
The TGA has currently accepted clinical data for the Novavax vaccine but has given no provisional approval, because it needs further phase 3 clinical trial data.
Even if it gains provisional approval, the TGA will still require its final clinical trial data and can cancel the provisional approval if it fails to do so.
Chief medical officer Paul Kelly said the TGA would “absolutely expedite” its consideration of Novavax, as it has for the Pfizer and AstraZeneca vaccines.
Moderna
Type: mRNA vaccine
Supply: none
Approval status: approved overseas, no application for TGA approval yet
Source: US and Europe
The Moderna vaccine is highly effective and is being rolled out across the United Kingdom and United States. Phase three trials showed a 94.1% efficacy against Covid-19 and 100% efficacy against severe Covid-19.
The UK began its rollout of Moderna in Wales this week.
Australia currently has no agreement to secure Moderna vaccine.
The Moderna product is also an mRNA vaccine, meaning Australia could not manufacture it onshore. Imports would be required.
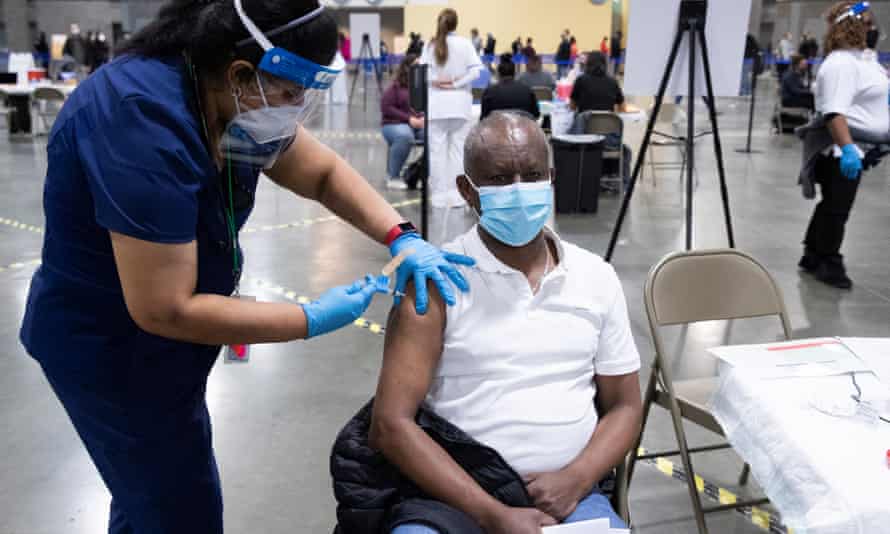
When asked if Australia would be seeking deals with new suppliers, Australia’s chief medical officer, Paul Kelly, said:
“All of those things are on the table. We are looking at all of those options right now.”
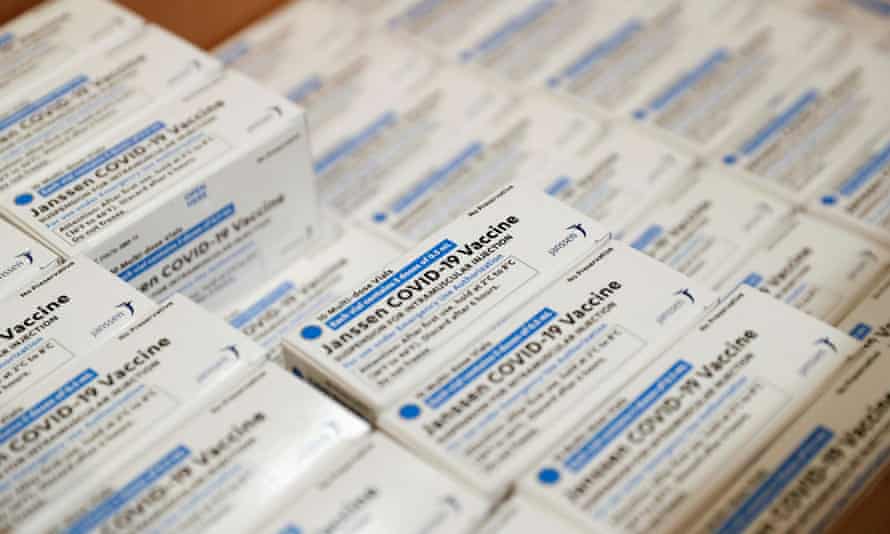
Johnson & Johnson
Type: viral vector vaccine
Supply: none
Approval status: approved overseas, TGA currently considering
Source: US and Europe
Johnson & Johnson’s vaccine is effective and enjoys a significant benefit over the others: it is a one-dose vaccine.
The viral-vector vaccine is currently approved for use in the United States and has been deployed widely in that country. Phase three trials showed it offered 66% protection against moderate Covid-19 disease and 85% protection against severe disease.
Australia has no agreement to secure the Johnson & Johnson vaccine.
The TGA has currently accepted clinical data for the Johnson & Johnson vaccine but has given no provisional approval. Even if it gains provisional approval, the TGA will still require its final clinical trial data and can cancel the provisional approval if it fails to do so.
The Covax facility
Australia potentially has access to a range of vaccines through what is known as the Covax facility, an international purchasing agreement.
The Covax facility requires each of the 188 member nations to contribute funding toward the development of vaccines. It then entitles them to a certain number of vaccines from a portfolio of manufacturers in return. The system is designed to ensure equitable access to vaccines across the world.
Sign up to receive the top stories from Guardian Australia every morning
Sign up to receive the top stories from Guardian Australia every morning
Australia is entitled to 25m doses through the Covax facility and gave an upfront payment of $123.2m.
It is unclear how close Australia is to securing any vaccines through Covax. It is reliant on receiving offers to purchase vaccines as they become available.
There are currently nine vaccines available that may become available through Covax which are made by AstraZeneca, Novavax, Moderna, Curevac, Sanofi, Inovio, Clover Biopharmaceuticals, Institut Pasteur, and the University of Hong Kong.
This content first appear on the guardian
